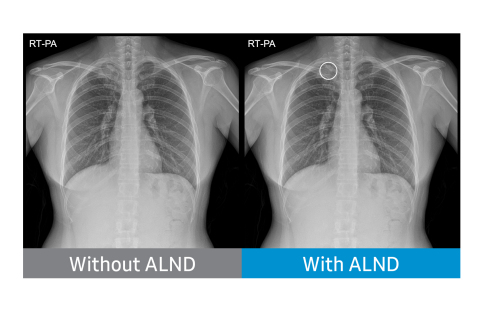
NeuroLogica, the healthcare subsidiary of Samsung, has received Food and Drug Administration (FDA) 510(k) clearance for its Auto Lung Nodule Detection (ALND) tool, the company said in a statement.
The offering provides an on-device, computer-assisted detection (CADe) solution for detecting pulmonary nodules from 10 to 30mm in size through an artificial intelligence (AI) algorithm.
The Auto Lung Nodule Detection (ALND) system is designed to aid the physician in reviewing PA chest radiographs of adults and is part of S-Station, an operation software installed on Samsung Digital X-ray Imaging systems, according to the company.
[Image courtesy: Samsung]